-
Reverse Osmosis
-
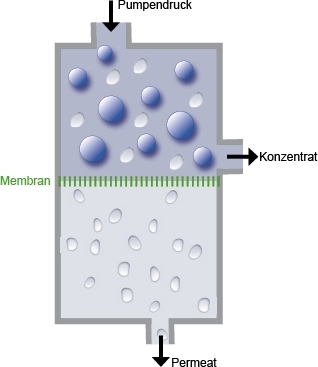 Principle:
Principle:
The osmosis is a physical process of the concentration equilibrium of two liquids of
different ion concentrations. This exchange take place via semi-permeable membranes.
Their physical properties are limited largely to the size of water molecules. The ions
of these liquids are balanced out only until no other natural ion exchange occurs, or on
the more concentrated side a pressure is built up, the so-called osmotic pressure. The
ions exchange in a gradient from the high concentrated to low concentrated Side.
-
In reverse osmosis, the ion-exchange occurs from the high concentrated to the low concentrated
side, as found in todays water treatment.
 Principle:
Principle:


